Peer Reviewed Results: Conformis Patient Specific iTotal CR Achieves Better Rotational Alignment and Tibial Fit Compared to Off-the-Shelf Implants
Manuscript highlights the intraoperative compromises of fit vs. rotation with the legacy off-the-shelf knee implants
BILLERICA, Mass., June 25, 2018 (GLOBE NEWSWIRE) — Conformis Inc. (NASDAQ:CFMS), a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture and sell patient specific joint replacement implants designed to fit each patient’s unique anatomy, today announced publication of a study showing that patients treated with the iTotal CR Knee Replacement Systems achieved better tibial fit and tibial rotational alignment compared to patients treated with three different off-the-shelf (OTS) total knee arthroplasty (TKA) products. Results of the study titled “In Vivo Tibial Fit and Rotational Analysis of a Customized, Patient-Specific TKA versus Off-the-Shelf TKA” were presented in the May 2018 issue of The Journal of Knee Surgery, a leading peer-reviewed orthopedic journal.
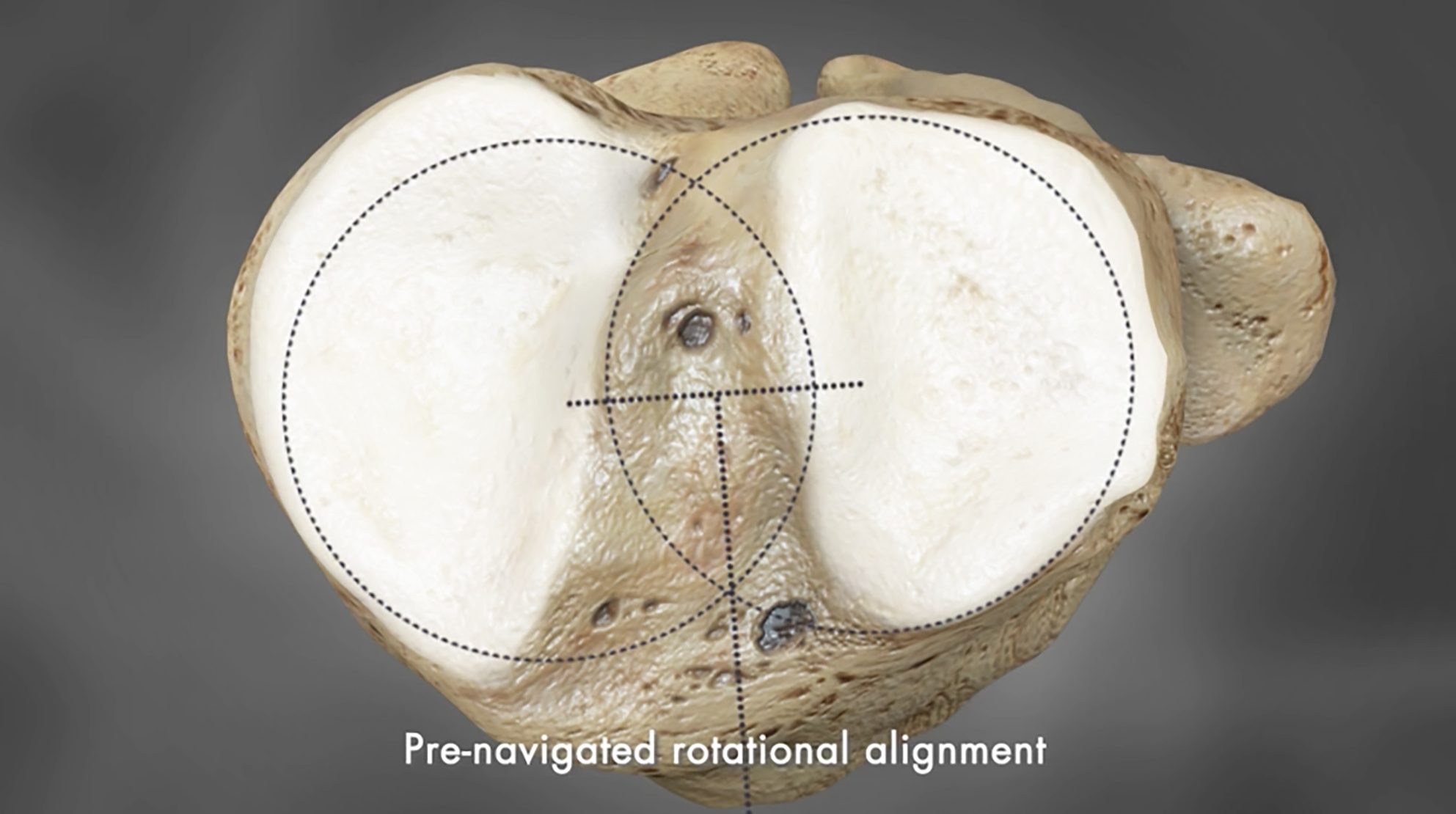
The study, which was conducted jointly by Greg Martin, MD in Boynton Beach, Florida and Lennart Schroeder, MD candidate Julius-Maximilians-Universitaet Wuerzburg, Germany, intraoperatively compared component fit in four tibial zones. The Conformis iTotal CR implant system and three OTS cruciate retaining TKA products including Biomet Vanguard, Zimmer NexGen and DePuy Sigma were evaluated intraoperatively in a total of 44 knees. Each implant system was evaluated intraoperatively on the same 44 knees to compare each brand’s optimal tibial fit. Component rotation was then evaluated post operation using CT-based imaging converted into computer aided design (CAD) models. Using these models, simulated surgery was performed with the OTS TKAs.
“One of the compromises and decisions we surgeons often need to make intraoperatively is the best method to achieve an optimal “fit” for the patient while maintaining proper rotational alignment,” says board certified, fellowship trained hip and knee surgeon, Gregory Martin, MD. “The real benefit that I’ve found using Conformis implants is that this compromise is completely obviated. The implants fit precisely as designed with the in-built rotation, and enough relief to allow me to fine tune my rotation based on the specific patient’s anatomy. This is borne out in our results.”
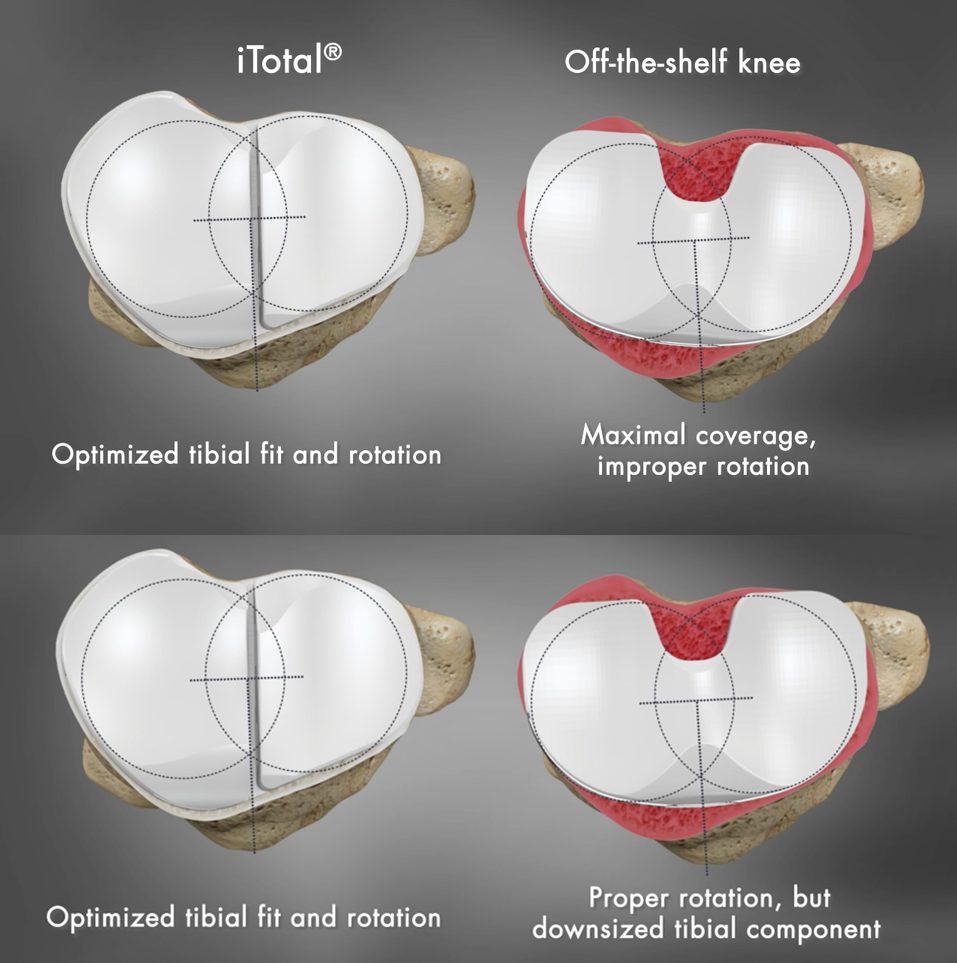
The surgeon placed all implants with proper rotational alignment and the results of this study show: 37% of OTS knees showed >3mm under-coverage compared to 18% of Conformis iTotal and 18% of OTS had an overhang of >3mm compared to 0% for iTotal. In previous studies1, component overhang of greater than 3mm was linked to significantly higher post-operative pain. Additionally, when the same 44 knees were positioned for optimal fit, 45% of OTS knees had rotational errors of >5 degrees and 4% had >10 degrees. Excessive internal tibial component rotation has been found to be one of the leading factors of residual pain in TKR and can be a source of functional deficit2. No rotational deviation was observed with the Conformis iTotal implants, which are pre-aligned rotationally to match the patient’s unique anatomy.
“This is the first study to evaluate both the fit of the tibial tray and rotation of our patient-specific designed implants compared with off-the-shelf implants,” said Mark Augusti, chief executive officer and president of Conformis. “We often hear from surgeons that when they use off-the-shelf implants they need to compromise either on fit or rotation in the operating room. The results of this new study demonstrate that the Conformis iTotal CR can help surgeons address both issues at the same time. This study adds to the growing body of evidence that the Conformis iTotal CR can lead to better outcomes for patients.”
Mr. Schroeder’s research and internship is funded by Conformis Inc. Dr. Martin was reimbursed by Conformis during the period of the study.
*Graphics are for demonstration purposes only and are not representative of a particular brand of off-the-shelf knee
About Conformis, Inc.
Conformis is a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture and sell joint replacement implants that are individually sized and shaped, or customized, to fit each patient’s unique anatomy. Conformis offers a broad line of customized knee implants and customized pre-sterilized, single-use instruments delivered in a single package to the hospital. In clinical studies, Conformis iTotal CR demonstrated superior clinical outcomes, including better function and greater patient satisfaction, compared to traditional, off-the-shelf implants. Conformis owns or exclusively in-licenses issued patents and pending patent applications that cover customized implants and customized patient-specific instrumentation for all major joints.
For more information, visit www.conformis.com. To receive future releases in e-mail alerts, sign up at http://ir.conformis.com/.
1 Mahoney OM, Kinsey T. Overhang of the femoral component in total knee arthroplasty: risk factors and clinical consequences. J Bone Joint Surg Am 2010;92(05):1115–1121
2 Nicoll D, Rowley DI. Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br 2010;92(09):1238–1244
Cautionary Statement Regarding Forward-Looking Statements
Statements in this press release about our future expectations, plans and prospects, as well as other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” and similar expressions, constitute forward-looking statements within the meaning of the safe harbor provisions of The Private Securities Litigation Reform Act of 1995. You should not place undue reliance on our forward-looking statements. Actual results could differ materially from the projections disclosed in the forward-looking statements we make as a result of a variety of risks and uncertainties, including risks related to our estimates and expectations regarding our results of operations, and the other risks and uncertainties described in the “Risk Factors” sections of our public filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent our views as of the date hereof. We anticipate that subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date hereof.
CONTACT:
Kelly Wakelee
kwakelee@berrypr.com
(212)-253-8241
Investor Contact:
Oksana Bradley
ir@conformis.com
(781) 374-5598
Photos accompanying this announcement are available at:
http://www.globenewswire.com/NewsRoom/AttachmentNg/e97b5a4e-0af6-4e8e-ae47-f0b8515bd2a2
http://www.globenewswire.com/NewsRoom/AttachmentNg/926a54ed-e68a-4475-8992-57c5060b41f5
The photos are also available at Newscom, www.newscom.com, and via AP PhotoExpress.
The design and placement of Conformis implants are developed for each patient using their specific anatomy.
Conformis Patient Specific iTotal CR Achieves Better Rotational Alignment and Tibial Fit Compared to Off-the-Shelf Implants
Related Posts
iTotal
Os únicos implantes totais de joelho realmente personalizados (específicos para cada paciente) com componente femoral CR (com preservação do LCP) e componente femoral PS (com estabilização posterior)
iTotalSobre a Conformis
Começamos com uma ideia simples: fazer com que o implante se encaixe ao paciente, ao invés de forçar o paciente a se encaixar ao implante
Sobre a Conformis