No more compromises on femoral fit
One of the primary drivers of patient dissatisfaction is residual, post-operative pain attributable to poor femoral component fit. In fact, one study of 437 TKR femoral components found that overhang ≥3mm is correlated with a 1.9x increase in the risk of post-operative pain. That same study found that overhang ≥3mm affected 57% of patients. Finally, 27% of the clinically important pain among all knees in the study was attributable to femoral overhang.1
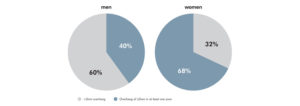
Prevalence of overhang in a cohort of men (176 knees) and women (261 knees)
Other total knee systems have tried to address this issue by adding more size options. Even with this advancement it’s virtually impossible to fit the unique shapes and sizes of patients’ femurs.
Patient-matched anatomic femur to avoid sizing compromises
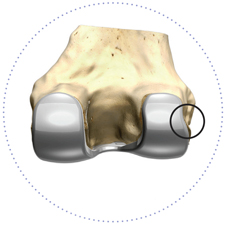
Popliteus tendon relief
Rather than using a variety of implant sizes to try and fit all patients, Conformis designs customized implants that fit all patients (within certain regulatory specs), regardless of their varying A/P and M/L sizes. Based on CT-scan data, these customized implants provide a patient-matched anatomic femoral component specific to the size and shape of each patient’s femur. This personalized fit is so precise that it virtually eliminates the sizing compromises typical in traditional, off-the-shelf TKAs that may lead to residual pain and patient dissatisfaction.
Personalized fit can also help avoid soft tissue issues (e.g. popliteus tendon “popping”) which would traditionally require releases to correct.
The iTotal CR Knee Replacement System is intended for use as a total knee replacement in patients with knee joint pain and disability whose conditions cannot be solely addressed by the use of a prosthetic device that treats only one or two of the three knee compartments, such as a unicondylar, patellofemoral or bicompartmental prosthesis. Only a licensed physician can help you determine the appropriate medical treatment. There are potential risks to knee replacement surgery, and individual results may vary. Before making any decisions concerning medical treatment, consult your physician regarding your options and the risks of those options. The longevity, performance and feel of any knee implant will depend on various factors, including your physical condition, your activity level, adherence to your physician’s instructions, and other factors.
The Indications for Use include:
- Painful joint disease due to osteoarthritis, traumatic arthritis, rheumatoid arthritis or osteonecrosis of the knee.
- Post traumatic loss of joint function.
- Moderate varus, valgus or flexion deformity in which the ligamentous structures can be returned to adequate function and stability.
- Failed osteotomies, hemiarthroplasties, and unicondylar, patellofemoral or bicompartmental implants.
- Revision procedures provided that anatomic landmarks necessary for alignment and positioning of the implant are identifiable on patient imaging scans.
The implant is intended for cemented use only.
Caution: Federal law restricts this device to sale by or on the order of a physician.



